
Peripartum depression is a serious mental health condition that can affect women during pregnancy or within the first year after childbirth. Although this period is often expected to be joyful, many women experience persistent sadness, anxiety, exhaustion, or emotional numbness that does not resolve on its own. When these symptoms interfere with daily functioning, emotional recovery, care of the baby, or bonding, professional care becomes essential. One treatment option that is sometimes discussed in this context is TMS for peripartum depression.
Transcranial Magnetic Stimulation, or TMS, is not considered a first-line treatment for most women. However, it may be explored in specific situations, particularly when standard treatments are not well tolerated or have not provided adequate relief. Understanding where TMS fits into care can help women make informed and thoughtful decisions about their mental health.
Understanding Peripartum Depression
Peripartum depression refers to major depressive episodes that occur during pregnancy or within the first year after delivery. Unlike temporary mood changes or the baby blues, peripartum depression is more intense, lasts longer, and significantly affects emotional and physical wellbeing.
Symptoms may include:
- Persistent low mood or sadness
- Loss of interest in activities
- Fatigue beyond normal pregnancy or postpartum tiredness
- Changes in sleep or appetite
- Difficulty concentrating or making decisions
- Feelings of guilt or worthlessness
- Anxiety or intrusive thoughts or images
For many women, these symptoms can be especially distressing as they adjust to the emotional and physical demands of pregnancy or early motherhood.The National Institute of Mental Health (NIMH) recognizes peripartum depression as a medical condition influenced by hormonal shifts, stress, prior mental health history, and social factors. It does not reflect weakness or failure.

Why Treating Peripartum Depression Can Be Complicated
Treatment decisions during pregnancy or the postpartum period often involve added layers of concern. Women may face challenges such as:
- Worries about the effects of antidepressant medication on a developing fetus
- Concerns about medication exposure while breastfeeding
- Previous experiences with medication side effects or limited benefit
- Persistent symptoms despite psychotherapy or other standard treatments
These factors can leave women feeling uncertain about next steps. In this setting, non-medication approaches such as TMS therapy may be discussed as part of a carefully guided and individualized treatment plan.
What Is TMS and How It Works
Transcranial Magnetic Stimulation (TMS) is a non-invasive treatment that uses targeted magnetic pulses to stimulate areas of the brain involved in mood regulation. Sessions do not require anesthesia, and individuals remain awake and alert throughout treatment. A typical course involves sessions five days a week over several weeks.
TMS is approved by the U.S. Food and Drug Administration (FDA) for the treatment of major depressive disorder, particularly for individuals who have not responded adequately to antidepressant medications. Because it does not involve systemic medication, TMS does not circulate through the body in the same way as traditional antidepressants. This is one reason it may be discussed with women who have concerns about medication exposure during pregnancy or the postpartum period.
Can TMS Be Used for Peripartum Depression?
While TMS is FDA approved for major depressive disorder, it has not received specific FDA approval for peripartum depression. In clinical practice, this means its use during pregnancy or postpartum may be considered off label. Off label use is common in medicine and reflects a clinician’s judgment when emerging evidence suggests potential benefit.
A review study examined the use of repetitive TMS (rTMS) during pregnancy and the postpartum period. The authors reported that available studies and case series showed improvement in depressive symptoms for many participants, with a generally favorable tolerability profile. The review also emphasized the importance of careful screening, informed consent, and collaboration between psychiatric and obstetric providers, while noting that larger controlled studies are still needed.
These findings suggest that TMS may be considered in selected cases, particularly for women whose symptoms have not improved with psychotherapy or medication, or when medication risks are a significant concern, including those whose depressive symptoms emerge or continue into the postpartum period.
Safety and What We Know So Far
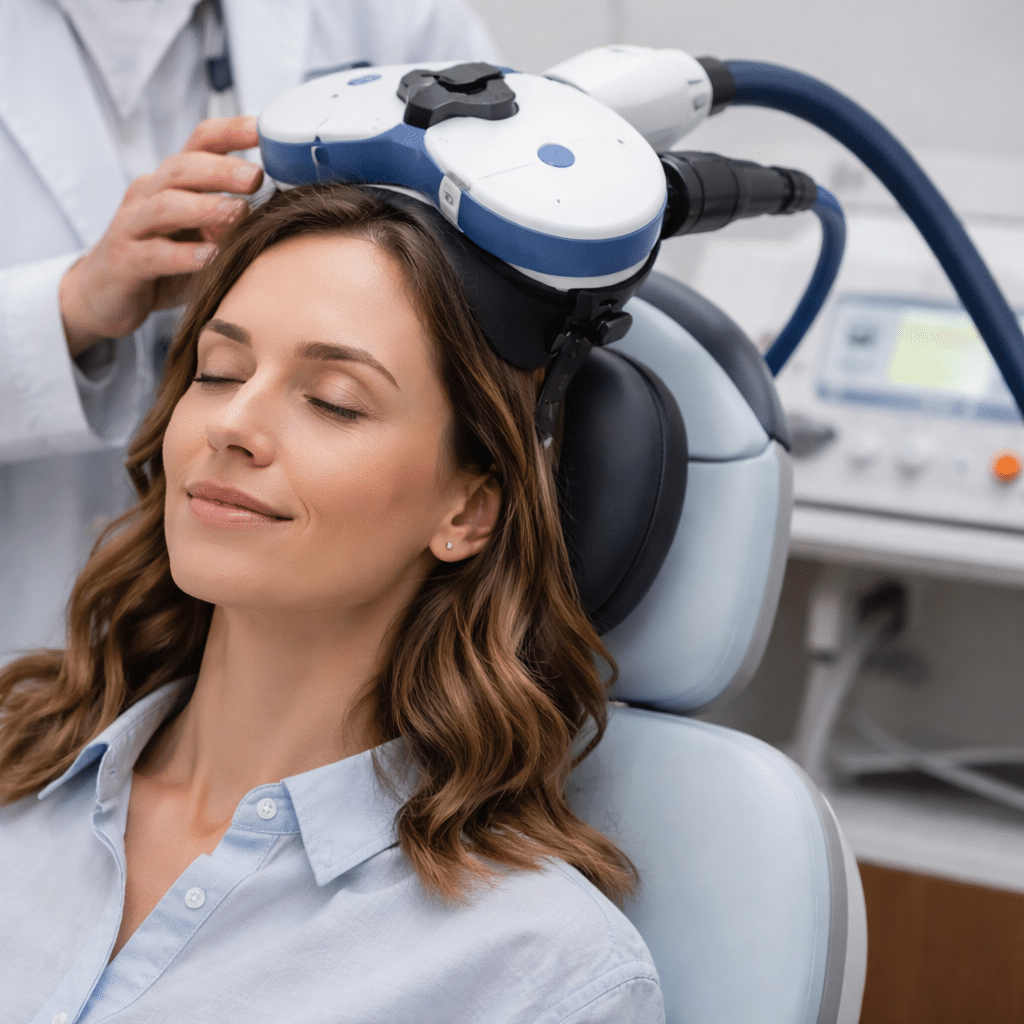
Current evidence suggests that TMS is generally well tolerated. The most commonly reported side effects include mild scalp discomfort or headaches during treatment, which often lessen over time. Serious adverse effects are rare.
Because TMS does not involve medication, it avoids many concerns related to fetal or infant exposure. However, absence of medication does not automatically mean a treatment is appropriate for everyone. Careful screening, ongoing monitoring, and shared decision-making remain essential. Importantly, untreated peripartum depression itself carries risks, including impaired bonding, increased stress, and poorer outcomes for women and their families.
Final Thoughts
If you are experiencing symptoms of peripartum depression, seeking professional support early can make a meaningful difference. Help is available whether symptoms arise during pregnancy or after birth. A qualified mental health professional can assess symptom severity and discuss evidence based options, including psychotherapy, medication, TMS, or a combination of approaches.
From a clinical perspective, the goal is not to promote a single solution, but to help each woman find relief in a way that prioritizes safety, dignity, and long term wellbeing. For some, TMS may become part of that path. For others, more traditional treatments may be sufficient. What matters most is that depression is recognized and treated with compassion, so women can heal and move forward in their lives and, when applicable, in their role as mothers.
Frequently Asked Questions (FAQs)
1. Is TMS safe during pregnancy or after childbirth?
TMS is generally well tolerated and does not involve medication, which is why it may be discussed during pregnancy or the postpartum period. Its use should always involve careful evaluation by a qualified mental health professional.
2. Is TMS FDA approved for peripartum depression?
TMS is FDA approved for major depressive disorder. While it does not have specific FDA approval for peripartum depression, clinicians may consider its use in certain situations based on available evidence, individual clinical factors, and careful risk-benefit assessment.
3. Can TMS replace antidepressant medication?
TMS is not intended to replace all other treatments and is typically considered when medications are not effective or not well tolerated. Treatment decisions should always be individualized.
4. How long does a typical TMS treatment course last?
A standard course of TMS usually involves sessions five days a week over several weeks. The exact duration can vary depending on individual response.
Responsibly edited by AI
Other Blog Posts in
Animo Sano Psychiatry is open for patients in North Carolina, Georgia and Tennessee. If you’d like to schedule an appointment, please contact us.
Get Access to Behavioral Health Care
Let’s take your first step towards. Press the button to get started. We’ll be back to you as soon as possible.ecovery, together.





